Additional doses for people 5 years and older with moderate to severe immune compromise
Wednesday Mar 30th, 2022
The Baltimore City Health Department recommends that people 5 years and older who are moderately to severely immunocompromised receive a 3 dose primary series (if vaccinated with Moderna/Pfizer) or a 2 dose primary series (if vaccinated with Johnson & Johnson) according to the Centers for Disease Control Interim Clinical Considerations for COVID-19 vaccination.
Individuals 12 years and older who are moderately to severely immunocompromised should also receive 2 booster doses at least 4 months apart.
An additional mRNA vaccine and two booster doses should be offered to individuals with moderate to severe immunocompromising conditions and/or taking immunosuppressive medication or treatments. These conditions and medications/treatments include, but or are not limited to:
Active treatment for solid tumor and hematologic malignancies
Receipt of solid-organ transplant and taking immunosuppressive therapy
Receipt of CAR-T-cell or hematopoietic stem cell transplant (within 2 years of transplantation or taking immunosuppression therapy)
Moderate or severe primary immunodeficiency (e.g., DiGeorge syndrome, Wiskott-Aldrich syndrome)
Advanced or untreated HIV infection
Active treatment with high-dose corticosteroids (i.e., ≥20mg prednisone or equivalent per day), alkylating agents, antimetabolites, transplant-related immunosuppressive drugs, cancer chemotherapeutic agents classified as severely immunosuppressive, tumor-necrosis (TNF) blockers, and other biologic agents that are immunosuppressive or immunomodulatory.
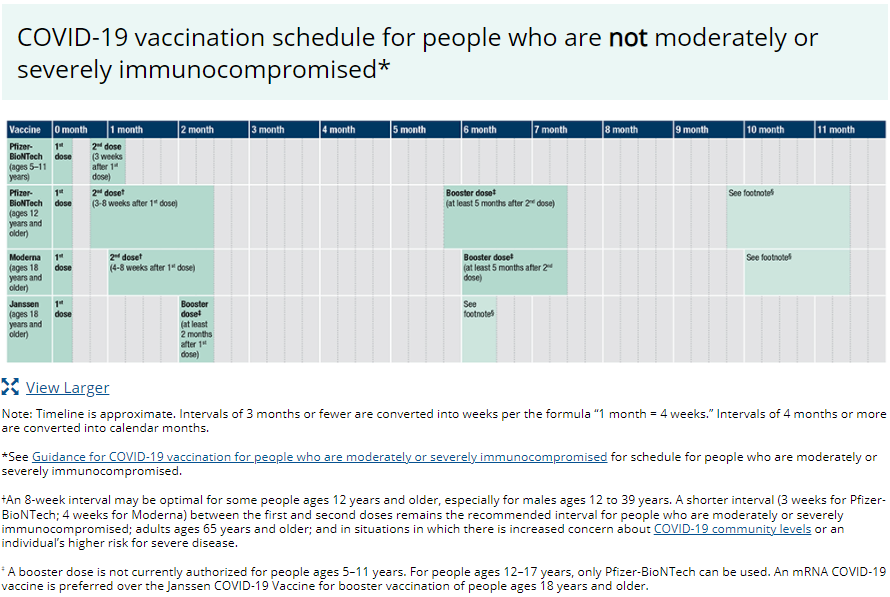
COVID-19 vaccination schedule for people who are not moderately or severely immunocompromised*
Individuals meeting the above criteria and vaccinated with Moderna or Pfizer need 3 primary series doses (dose 1, dose 2, and dose 3/additional dose). There should be at least 28 days between dose 2 and dose 3/additional dose.
Individuals meeting the above criteria and vaccinated with Johnson & Johnson need 2 primary series doses (dose 1 and dose 2/additional dose). There should be at least 28 days between dose 1 and dose 2/additional dose. The mRNA vaccine, either Pfizer or Moderna is preferred for dose 2/additional dose in those initially vaccinated with Johnson & Johnson.
People who are 12 years old and up and moderately to severely immunocompromised should also receive two booster doses, in addition to the primary series doses. Individuals vaccinated with Moderna or Pfizer should get the first booster at least 3 months after completion of the primary series. The second booster should be given at least 4 months after the first booster. Individuals vaccinated with Johnson & Johnson should receive the first booster at least 2 months after completion of the primary series. The second booster should be given at least 4 months after completion of the first booster.
Serologic testing and cellular immune testing prior to administration of the additional dose is not recommended at this time. Baltimore City Health Department will not require a medical provider’s note or prescription for the administration of an additional dose. Resident self-attestation of having a moderate to severe immunocompromising condition qualifying them for an additional dose will be honored.
Based on the evidence showing a limited immune response after COVID-19 vaccination in this population, providers should recommend individuals with moderate to severe immunocompromising conditions continue to practice non-pharmaceutical interventions - wearing a mask, remaining 6 feet apart from others, avoiding large crowds, and poorly ventilated areas. Providers should strongly recommend COVID-19 vaccination for close contacts of individuals with immunocompromising conditions.
We recommend residents with immunocompromising conditions or taking medication that suppresses the immune system talk with their clinical provider about the need for an additional dose of the mRNA COVID-19 vaccine. Clinical providers are best equipped to make informed decisions about their patient's medical conditions and the timing of the additional dose of the mRNA vaccine.
The Baltimore City Health Department will continue to monitor updates, and adjust guidance, as more information arises.